FDA Approves first ever Type 1 diabetes delaying drug
November 17, 2022 the day when the world got it’s first ever type 1 diabetes delaying drug. Type 1 diabetes is also known as insulin-dependent diabetes. In this condition, the pancreatic cells produce little or no more insulin to maintain blood glucose levels. The exact cause of type 1 diabetes is unknown. But the most common cause is the defective immune response. During this, the body’s immune system starts destroying the insulin-producing cells in the pancreas. In a recent study, the United States Food and Drug Administration approved a type 1 diabetes delaying drug.
This modern era drug is known as Tzield. It is available in injectable form with a combination of two drugs. These drugs have the capacity to delay the onset of type I diabetes in adults at the stage and younger children at stage 2. The Food and Drug Administration approved this drug to treat such diabetic patients who are at higher risk. This is the first ever type 1 diabetes delaying drug approved.
Teplizumab or Tzield was under clinical trials for decades. After the success of this recent clinical trial, the FDA approved this drug for use in patients. This clinical trial was double-blinded, randomized, and placebo controlled. In this study, 76 patients were selected with type 1 diabetes at stage 2. Patients were on a follow-up period of about 51 months.
The studies confirmed that 44% of people receiving teplizumab continue to develop type 1 diabetes at stage 3 relative to the 72% of placebo patients. Whereas, those patients on drugs who also developed the disease are at a median of 25 months before the onset of stage 3. Below we talk about the first ever type 1 diabetes delaying drug in details.
Why is there a need to delay type 1 diabetes?
Let’s have a look at why type 1 diabetes delaying drugs are needed. Type 1 diabetes can start from an early age even in childhood. Suppose an eight-year-old child is having type I diabetes. These type 1 diabetes delaying drugs can affect the time at which you must consider regulating and maintaining your blood glucose levels. These type 1 diabetes delaying drugs can delay the severity of type 1 diabetes up to two years. And that’s gross.
This extra time helps younger patients to maintain the pressure of such diseases. Moreover, blood exposure to high blood glucose for a lesser time period may reduce the chances of health complications.
Researchers also suggested improvements in these drugs regarding their efficacy and efficiency of the drugs. These type 1 diabetes delaying drugs are a huge benefit for the patients suffering from the risks of type I diabetes.
What is the mechanism of insulin to regulate body glucose levels?
Insulin is a vital hormone released from the pancreas’s beta cells. These beta cells are present within a cellular group known as islets of Langerhans. These produce a larger amount of insulin against the stimulus of increased blood glucose levels. Insulin is delivered from the blood plasma to all the muscle cells to utilize glucose. In this way, insulin regulates the blood glucose levels at optimum levels.
People who lack insulin may suffer from increased blood glucose levels or hyperglycemia. It can disturb the body’s metabolism and other normal functions of tissues and organs.
Type 1 diabetes
Type 1 diabetes usually gets diagnosed during childhood or adulthood. It can appear at any age. A familial history of type 1 diabetes may be a risk factor to induce type 1 diabetes. Stage 1 indicates that phase when two or more autoantibodies concerning beta cells are present in the body. But, in this phase, the blood glucose levels are normal. Stage 2 indicates high blood glucose levels but remains asymptomatic. Stage 3 is when the clinical diagnosis occurs and the disease may become overt. You should also be aware of the signs of diabetes complications.
The most common symptoms of type 1 diabetes include increased thirst, hunger, frequent urination, weight loss, and different metabolic problems. Type 1 diabetes can be prevented by proper diet and regular exercise but insulin is mandatory to control blood glucose levels. Insulin is administered through regular injections to overcome the demand for insulin in the body.
Teplizumab
Teplizumab is a monoclonal antibody under the name of Tzield. This is the first type 1 diabetes delaying drug approved by the FDA. This medication was redeveloped by the ProventionBio. It is an antibody that binds to the CD3 marker molecules present on the outer surface of specific defense cells. Its mechanism depends on the binding capacity of the drug. It deactivates the autoimmune destructive cells to prevent the destruction mechanism of beta cells. It binds through a weak union to the T-cell receptor CD3 complex. This union stimulates a signaling pathway.
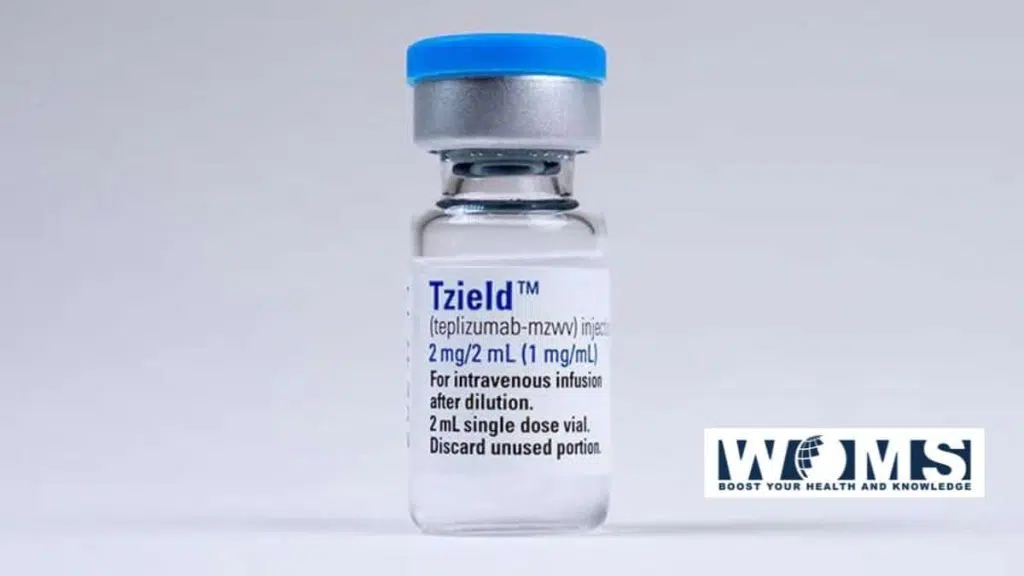
This mechanism causes the inactivity or cell death of the activated T-effector cells. In this way, it increases the proportion of the regulatory T-cells and regulatory cytokines. In addition, it moderates the immune response for the redevelopment of immune tolerance. Tzield is available in the injectable formulation and must be injected intravenously once per day for almost two weeks.
What are the adverse effects of Teplizumab?
Teplizumab or Tzield can cause a severe decrease in the lymphocyte count. The decreased lymphocyte count may lead to multiple infections in such patients. In addition, it can stimulate a larger amount of inflammatory cytokines.
These cytokines can trigger multi-organ disorders and systemic hyperinflammation. These patients must take precautionary medications to regulate cytokine release before receiving the injections of Teplizumab. To build a better immunity you should consume green fruits in a regularly basis. Check out the health benefits of green fruits.
Some patients may feel hypersensitivity to such drugs. The patient must be properly vaccinated before the start of treatment. Since it is a recently approved type 1 diabetes delaying drug, some side effects may be seen so precautions should be taken.
Conclusion
Type 1 diabetes is associated with a variety of health complications and risk factors. In addition, type 1 diabetes is usually diagnosed at a very early or young age. To prevent the burden of type 1 diabetes and associated risks, the researchers performed blind-ended clinical trials on Teplizumab. The FDA approved this drug to use in patients having type 1 diabetes. These drugs have the capability to slow down the process of type 1 diabetes. In this way, it helps the patients to lower some serious health-associated problems and became first ever approved type 1 diabetes delaying drug
Frequently asked questions (FAQs)
Is it safe to use type 1 diabetes delaying drugs?
Yes, it is safe to use these drugs to prevent the health hazards associated with type 1 diabetes. The FDA approved this drug by measuring its safety of this drug in multiple clinical trials on patients suffering from type 1 diabetes.
For how long, does this treatment take?
Teplizumab or Tzield is available in injectable form. It is usually for about two weeks or 14 days with a single dose every day.




